1) State and explain Henry's law.?
ans) Henry’s law states that at constant temperature, the solubility of a gas in a liquid is directly
proportional to the pressure of the gas.
Mathematically, p = KH.χ (where p is the partial pressure, χ is the mole fraction and KH is the Henry’s law
constant).
2) what are colligative properties?
ans)These are properties which depend only on the number of solute particles and not on their nature.
3) what is meant by osmotic Pressure?ans) It is the excess pressure that must be applied on the solution side to prevent osmosis.
4) What is reverse osmosis? Write any one of its applications.?
ans) : It is the process of flow of solvent molecules from solution side to solvent side through a semi
permeable membrane (SPM), when a pressure greater than osmotic pressure is applied on the solution
side. [OR, If a pressure larger than the osmotic pressure is applied to the solution side, the direction of
osmosis gets reversed]. It is used in desalination of sea water OR in water purification.
5) what are isotonic solutions?
ans) Two solutions having the same osmotic pressure are called isotonic solutions. E.g. 0.9% (mass/volume)
NaCl solution and our blood cells.
6) . what is abnormal mol. Weight?.
ans) Abnormal molar mass occurs when the experimentally determined molar mass of a substance using colligative properties differs from its theoretically expected molar mass, primarily due to solute association (leading to higher observed molar mass) or dissociation (leading to lower observed molar mass) in solution.
7) what are electrochemical cell? Give examples.
ans) An electrochemical cell is a device that either generates electrical energy from chemical reactions or uses electrical energy to drive chemical reactions. Essentially, they convert chemical energy into electrical energy, or vice versa, through redox (reduction-oxidation) reactions.
8) Write the representation of a Daniel cell. Also write its anode reaction, cathode reaction and net reaction?
ans) Representation of Daniel cell: Zn|Zn2+||Cu2+|Cu
Anode reaction: Zn → Zn2+ + 2 e–
Cathode reaction: Cu2+ + 2 e- → Cu
Net reaction: Zn + Cu2+ → Zn2+ + Cu
: Ecell = E*cell + 0.0591/2
log [𝐶𝑢2+]/ [𝑍𝑛2+]
10) State Kohlarausch's law?
ans) It states that the limiting molar conductivity of an electrolyte is the sum of the individual contributions
of the anion and the cation of the electrolyte.
Application: It is used to calculate the limiting molar conductivity of any electrolytes.
11) Write the anode and cathode reactions occur in the operation of a lead storage battery. Mention the
electrolyte used in the battery.?
ans) Anode reaction: Pb + SO4 2− → PbSO4 + 2e–
Cathode reaction: PbO2 + SO4 ^2− + 4H+ + 2e– → PbSO4 + 2H2O
Net reaction: Pb + PbO2 + 2H2SO4 → 2PbSO4 + 2H2O
Electrolyte used is 38% H2SO4 .
12) What are fuel cells?
ans) Fuel cells are galvanic cells which convert the energy of combustion of fuels (like hydrogen, methane,
methanol etc.) directly into electrical energy.
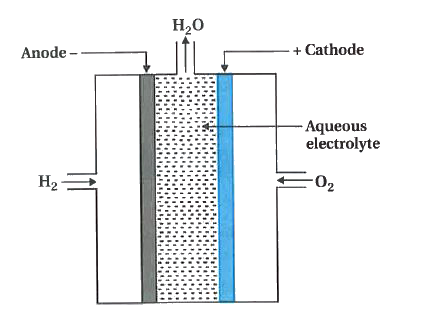
13) Diagrammatically represent H2 – O2 fuel cell. Write the anode reaction, cathode reaction and overall cell
reaction taking place in it?
ans)
👈Anode reaction: 2H2(g) + 4OH–(aq) → 4H2O(l) + 4e– Cathode reaction: O2(g) + 2H2O(l) + 4e– → 4OH–(aq)
Net Reaction: 2H2(g) + O2(g) → 2H2O(l)
14) Write the expression for integrated rate equation for a first order reaction.?
ans) K=2.303/t log[R]o/[R]
15) Write any four characteristic properties of transition elements.?
`ans) (i) They are metals (ii) show variable oxidation states (iii) form coloured ions (iv) most of them are
paramagnetic (v) show catalytic property.
55. Zinc (atomic number = 30) is not a transition.
16) Distinguish between order and molecularity?
ans)
| Order |
Molecularity |
| It is the sum of the powers of the concentration terms in the rate law expression. |
It is the total number of reactant species collide simultaneously in a chemical reaction. |
| It is an experimental quantity |
It is a theoretical quantity |
| It can be zero or fractional |
It cannot be zero or fractional |
17) Describe the method of preparation of potassium dichromate from chromite ore.
Ans) (i) Conversion of chromite ore (FeCr2O4) to sodium chromate (Na2CrO4) by fusing with sodium
carbonate in presence of air. (ii) Conversion of sodium chromate to sodium dichromate (Na2Cr2O7) by treating with sulphuric acid.
(iii) Conversion of sodium dichromate to potassium dichromate (K2Cr2O7) by treating with KCl.
18) How will you prepare KMnO4(Potassium permanganate) from MnO2?
ans) Potassium permanganate is prepared from MnO2 (pyrolusite) and it involves two steps:
(i) MnO2 is fused with KOH to form potassium manganate (K2MnO4).
(ii) Potassium manganate is acidified to form potassium permanganate (KMnO4).
19) What is Lanthanoid contraction? Give reason for it?`
ans) The regular decrease in the atomic and ionic radii along lanthanide series is known as lanthanide
contraction. It is due to the poor shielding effect of f – electrons and increase in nuclear charge.
❤ Uses of lanthanoid contraction?
ans) The main use of the lanthanoids is for the production of alloy steels. An important alloy is mischmetal which consists of lanthanoid metal (∼95%) and iron (∼5%) and traces of S, C, Ca and Al.A great deal of mischmetall is used in Mag-nesium based alloy to produce bullets
20) Write any two consequences of Lanthanoid contraction.?
ans) (i) 2nd and 3rd row transition series elements have similar radii.
(ii) Lanthanides have similar physical properties and they occur together in nature.
21) Draw the structures of chromate and dichromate ions.?
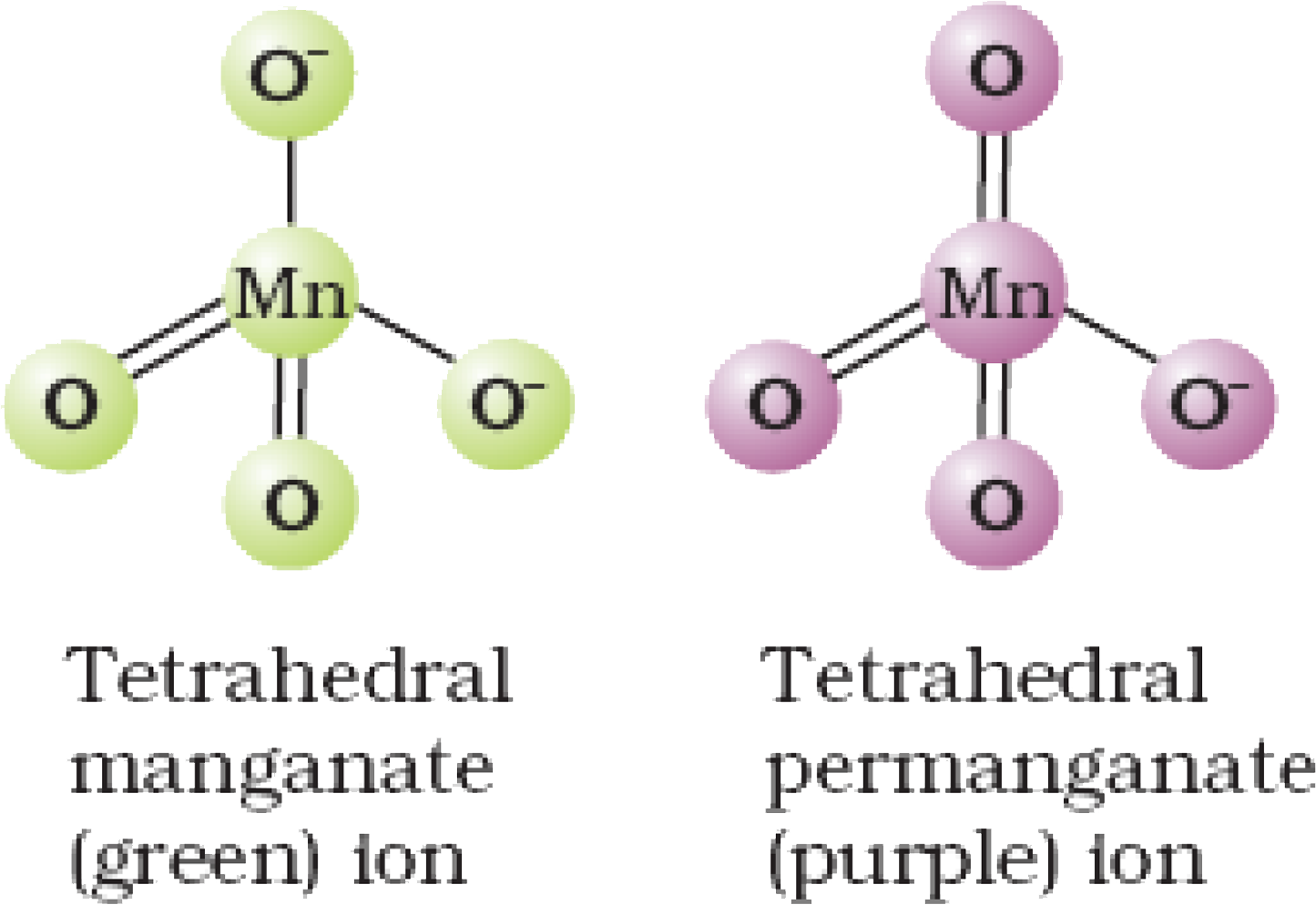
22) Draw the structures of mangante and permanganate ions?
23) Describe the four types of structural isomerism exhibited by co-ordination compounds. ans) Ionisation Isomerism: arises due to the exchange of ions between the inside and outside of co
ordination sphere.
E.g.: [Co(NH3)5SO4]Br and [Co(NH3)5Br]SO4.
ii) Linkage Isomerism: arises in a co-ordination compound containing ambidentate ligand, which can bound
to the central atom through more than one donor atoms.
E.g.: [Co(NH3)5(ONO)]Cl2 and [Co(NH3)5(NO2)]Cl2
iii) Co-ordination Isomerism: arises due to the interchange of ligands between cationic and anionic co
ordination entities.
E.g.: [Co(NH3)6][Cr(CN)6], and [Cr(NH3)6][Co(CN)6]
iv) Solvate Isomerism: arises due to the difference in the no. of solvent molecule bonded to the metal ion
as ligand. E.g.: [Cr(H2O)6]Cl3 and [Cr(H2O)5Cl]Cl2.H2O
23) Write any 4 postulates of Werner’s Co–ordination theory?
ans) (i) Every metal has two types of valencies – primary valencies and secondary valencies.
(ii) Primary valencies are ionisable, while secondary valencies are non–ionisable.
(iii) Primary valencies are always satisfied by negative ions, while secondary valencies may be satisfied by
negative ions or neutral molecules.
(iv) Primary valencies give the oxidation state of the metal, while secondary valencies give the co
ordination number of the metal.
24) Write any two limitations of valance bond theory of co-ordination compounds?
ans) (i) It involves a large number of assumptions.
(ii) It does not explain the colour exhibited by co-ordination compounds.
25) What is Tollen’s reagent and Fehling’s reagent. Explain how these reagents are used to distinguish
aldehydes and ketones.?
ans) Tollen’s reagent is ammoniacal silver nitrate and Fehling’s reagent is a mixture of aqueous CuSO4
(Fehling’s reagent A) and alkaline sodium potassium tartarate (Fehling’s reagent B).
Tollen’s test: When an aldehyde is heated with Tollen’ s reagent, we get a bright silver mirror.
Fehling’s Test: When an aldehyde is heated with Fehling’s reagent A and B, we get a red precipitate of
cuprous oxide (Cu2O).
26) What is aldol condensation reaction?
ans) Aldehydes or ketones having at least one α-hydrogen atom when heated with dilute alkali, we get
α,β–unsaturated aldehyde or ketone. This reaction is called Aldol condensation reaction.
27) What is Cannizzaro reaction?
ans) Aldehydes having no α–hydrogen atom, when treated with conc. Alkali, undergo self-oxidation and
reduction (disproportionation) to form alcohol and carboxylic acid salt. This reaction is called Cannizzaro
reaction.
2 HCHO (Formaldehyde)
Conc. KOH
→
CH3—OH (methanol)
+ H—COOK (potassium formate)
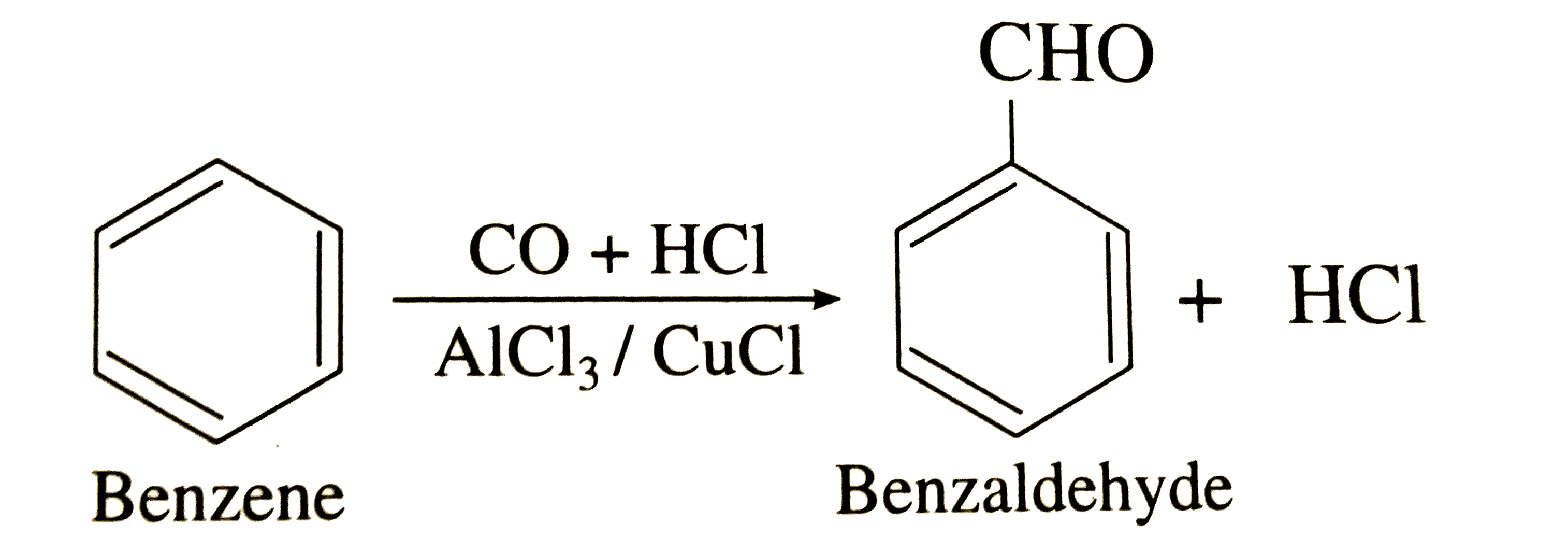
28) Gatterman–Koch reactionans) Gatterman–Koch Reaction: Benzene when treated with CO and HCl in the presence of anhydrous
aluminium chloride or cuprous chloride, we get benzaldehyde.
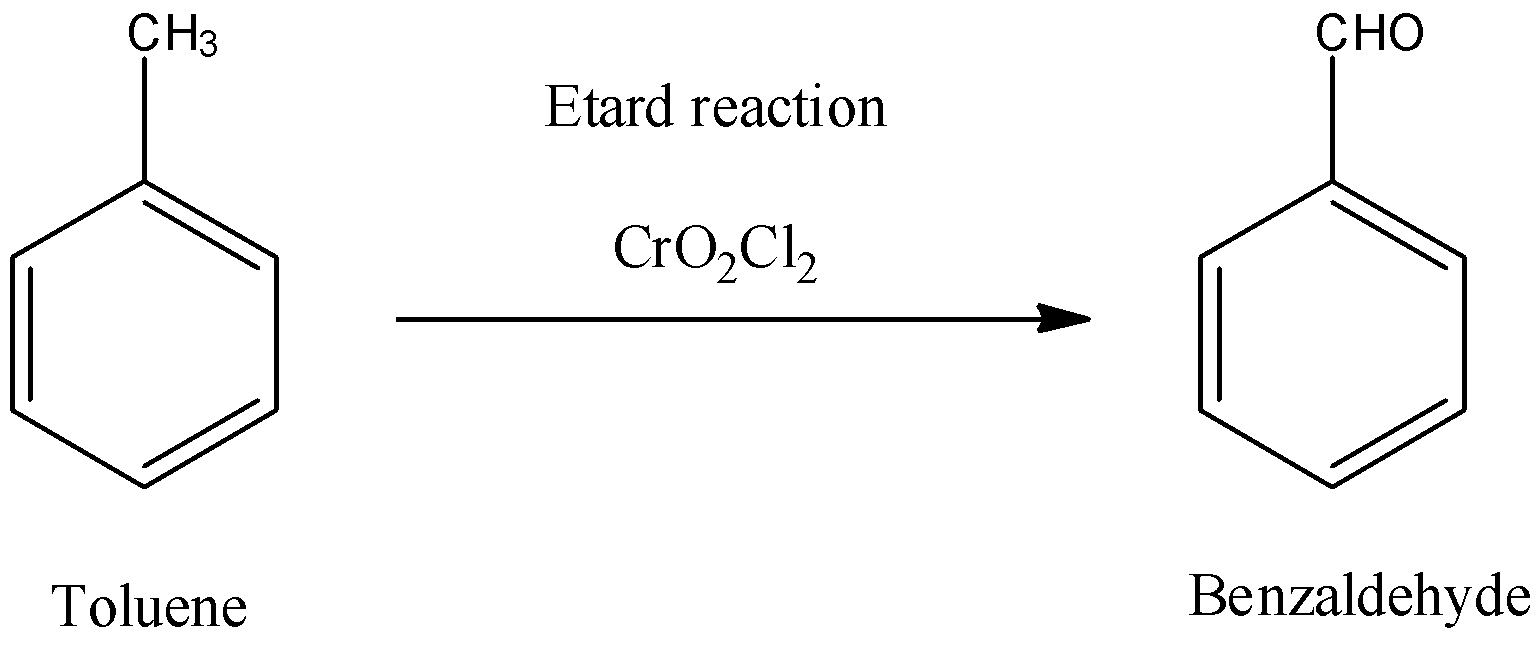
29) Etard Reaction
ans) When toluene is oxidised using chromyl chloride (CrO2Cl2) in CS2 followed by
acidification, we get benzaldehyde.
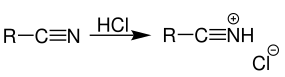
29) Stephen Reactionans) Nitriles when reduced using stannous chloride (SnCl2) and HCl followed by
acidification, aldehydes are formed.
30) Clemmensen Reduction:
ans) Clemmensen Reduction: Aldehydes and ketones when reduced using Zinc amalgam and Conc. HCl,
we get alkane.
Ethanal to Ethane Reaction
CH3—CHO (Ethanal)
Zn/Hg & Conc.HCl
→
CH3—CH3 (Ethane)
32) Wolff-Kishner Reductionans) Aldehydes and ketones when reduced using hydrazine followed by heating
with KOH in high boiling solvent like ethylene glycol, we get alkane.
33) Hell-Volhard-Zelinsky Reaction
34) What is Hinsberg’s reagent? How will you distinguish primary, secondary and tertiary amines using this
reagent?
ans) Hinsberg reagent is Benzenesulphonyl chloride (C6H5SO2Cl)
Primary amines react with Hinsberg reagent to form a precipitate (of N-alkylbenzenesulphonamide), which
is soluble in alkali.
Secondary amines react with Hinsberg reagent to give a precipitate (of N,N-dialkylbenzenesulphonamide),
which is insoluble in alkali.
Tertiary amines do not react with Hinsberg reagent
35) Write the carbyl amine reaction, which is used as test for primary amines.
ans) : Primary amines on heating with chloroform and alcoholic potassium hydroxide form foul smelling
isocyanides or carbylamines. This reaction is known as carbyl amine reaction or isocyanide test.
36) What is coupling reaction? Write one use of the product formed in this reaction.
ans) Benzene diazonium chloride when treated with phenol in basic medium, we get p-hydroxyazobenzene,
which is used as a dye.
37) How will you prepare p-nitroaniline from aniline?
ans) Aniline is first acetylated to get acetanilide, this on nitrating with nitration mixture followed by
acidification, we get p-nitroaniline.
38). Write a method to prepare Glucose from Starch.ans) Glucose is obtained by boiling starch with dilute H2SO4 at 393 K under pressure.
39) What is invert sugar?
ans) The product obtained after the hydrolysis of cane sugar is called invert sugar.
40) Write any three differences between DNA and RNA.
Propanone to Propane Reaction
CH3—CO—CH3 (Propanone)
(i) NH2—NH2
(ii) KOH/Ethylene glycol/Δ
→
CH3—CH2—CH3 (Propane)
DNA vs RNA Comparison
| DNA |
RNA |
| The pentose sugar is 2-deoxy ribose. |
The pentose sugar is ribose. |
| Double stranded. |
Single stranded. |
| The nitrogen bases present are Adenine, Guanine, Cytosine and Thymine. |
Instead of Thymine, Uracil is present. |
41) Write any two postulates of Werner's theory of
Co-ordination compounds?ans) Primary Valency ; Ionisable,non-directional,It gives the oxidation state
of the metal.It is always satisfied by(-ve
ions.
~Seconday valency ; non-ionisable,directional,It gives the co-ordination
number of the metal.It may be satisfied by -ve
ions or neutral molecules.
42) Write any three differences between SN1 and SN2 reactions.?
| SN1 Reaction |
SN2 Reaction |
| Occurs in two steps. |
Occurs in one step. |
| Order = 1 & molecularity = 1. |
Order = 2 & molecularity = 2. |
| Intermediate carbocation is formed. |
No intermediate is formed. |
43) What is Lucas reagent? Explain how this reagent helps to distinguish primary, secondary and tertiary
alcohols.
ans) Lucas reagent is a mixture of conc. HCl and anhydrous ZnCl2.
Lucas Test: Tertiary alcohols react with Lucas reagent and form immediate turbidity; secondary alcohols
form a turbidity within 5 minutes while primary alcohols do not form turbidity at room temperature. They
give turbidity only on heating.
44) Alcohols and phenols have higher boiling points. Why?
ans) Because of the presence of inter molecular hydrogen bonding in alcohol and phenol.
45) What is “Wood spirit”? Give its commercial preparation.
ans)Wood spirit is Methanol Or, Methyl alcohol.
It is commercially prepared by the catalytic hydrogenation of carbon monoxide at about 573-673 K
temperature, 200-300 atm pressure and in the presence of ZnO – Cr2O3 catalyst.
OR, the equation:
46) Explain a method for the manufacture of ethanol.
ans) By the hydration of ethene in acidic medium.
47) Explain the following:
a) Williamson’s synthesis
b) Esterification
c) Reimer-Tiemann Reaction
d) Kolbe’s Reaction
ans) Williamson’s synthesis: Alkyl halide reacts with sodium alkoxide to form ether. This reaction is
called Williamson’s ether synthesis.
E.g.: CH3-CH2Cl + CH3ONa → CH3-CH2-O-CH3 + NaCl
(Ethyl chloride) (Sod. Methoxide) (Ethyl methyl ether)
Esterification: Alcohols and phenols react with carboxylic acids, acid chlorides and acid anhydrides to
form esters.R-OH + R-COOH ⭢H+ R-COOR + H2O
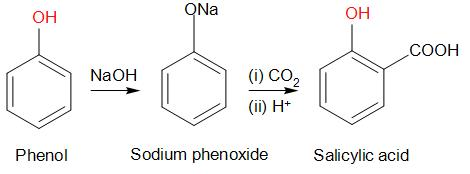
Kolbe’s Reaction: Phenol when treated with aqueous sodium hydroxide and CO2 followed by
acidification, we get salicylic acid (2-Hydroxybenzoic acid)47) How will you convert:
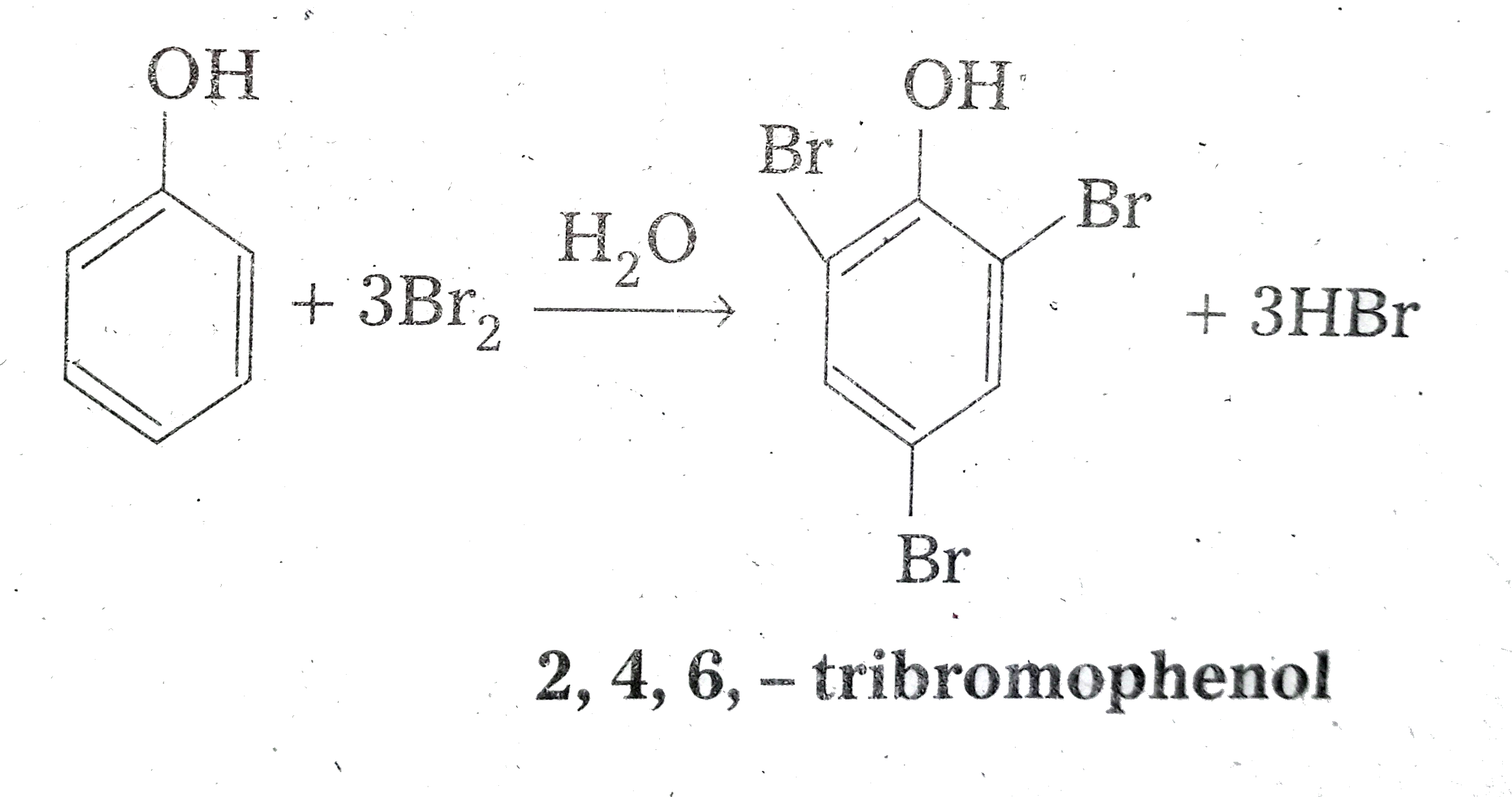
(i) Phenol to 2,4,6-tribromophenol
(ii) Benzene sulphonic acid to phenol
(iii) Aniline to phenol
(iv) Ethanol to ethanal
~(i) By treating with bromine water
(ii) Benzene sulphonic acid is treated with sodium hydroxide followed by acidification we get phenol.
(iii) Aniline on diazotisation to form benzene diazonium chloride which on warming with water we get
phenol.
(iv) Ethanol on oxidation using CrO3 (chromic anhydride), we get ethanal.
CH3-CH2-OH ⭢CrO3 CH3-CHO
48) Phenols are acidic. Why?~ans) (i) Due to the greater stability of the phenoxide ion formed.
(ii) Due to the greater electronegativity of sp2 hybridised carbon to which -OH group is bonded.
49) What is Tollen’s reagent and Fehling’s reagent. Explain how these reagents are used to distinguish
aldehydes and ketones.
ans)Tollen’s reagent is ammoniacal silver nitrate and Fehling’s reagent is a mixture of aqueous CuSO4
(Fehling’s reagent A) and alkaline sodium potassium tartarate (Fehling’s reagent B).
Tollen’s test: When an aldehyde is heated with Tollen’ s reagent, we get a bright silver mirror.
Fehling’s Test: When an aldehyde is heated with Fehling’s reagent A and B, we get a red precipitate of
cuprous oxide (Cu2O).
50) What is Hinsberg’s reagent? How will you distinguish primary, secondary and tertiary amines using this
reagent?
ans) Hinsberg reagent is Benzenesulphonyl chloride (C6H5SO2Cl)
Primary amines react with Hinsberg reagent to form a precipitate (of N-alkylbenzenesulphonamide), which
is soluble in alkali.
Secondary amines react with Hinsberg reagent to give a precipitate (of N,N-dialkylbenzenesulphonamide),
which is insoluble in alkali.
Tertiary amines do not react with Hinsberg reagent.
51) What is Hoffmann bromamide reaction? What is the significance of this reaction?
ans) Amides on treating with bromine and alcoholic NaOH to give amines. This reaction is known as
Hoffmann Bromamide degradation Reaction.
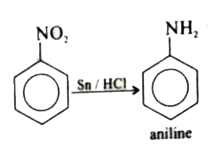
52) How will you convert nitrobenzene to aniline
ans) By reduction using iron and HCl or tin and HCl
53) What are pseudo first order reactions? Give one example.
ans) These are reactions which appears to follow higher order but actually follows first order kinetics.
E.g.: Hydrolysis of ester OR, inversion of cane sugar.
ALLOY FORMATION.ans) Alloys are homogeneous solid solutions of
elements in which at least one element is a
metal.
Because of similar radii and other charac
teristics of Transition metals, they readily
form alloys.
The alloys formed are hard and have high
m.p.
Examples: Bronze ( Cu,Zn), Stainless steel
( Fe,C,Ni,Mn and Cr ).
54) What are enzymes ?
- Enzymes are biocatalyst (catalyzes the chemical reactions of livings)
- Almost all enzymes are proteins.There are some nucleic acids that behave like enzymes, called ribozymes.
55) Explain the following reactions: a) Swarts reaction b) Sandmeyer’s reaction c) Wurtz–Fittig
reaction d) Fittig reaction e) Wurtz reaction.
ans) Swarts reaction: Alkyl chlorides or iodides react with AgF to form alkyl fluorides.
R–Cl + AgF → R–F + AgCl
*Sandmeyer’s reaction: Benzene diazonium chloride when treated with cuprous chloride and dil. HCl, we
get chlorobenzene.
Wurtz–Fittig reaction: A mixture of alkyl halide and aryl halide react with metallic sodium in dry
ether to give alkylarene.
Fittig reaction: Aryl halides react with metallic sodium in dry ether to give diaryl. Wurtz reaction: Alkyl halides react with metallic sodium in dry ether to form alkane. ans) An alloy contains lanthanide metal ~ 95%, iron ~ 5% and traces of S, C, Ca and Al
58) Explain manufacture of glucose
Ans: Commercially glucose is obtained by hydrolysis of starch by boiling it with dil.
H2SO4 at 393 K under pressure
59) What is denaturation of alcohol?
Ans: It is a method of making alcohol unfit for drinking by adding copper sulfate and pyridin
60) what is collision theory?
ans) The collision in which collide with sufficient K.E and proper orientation so as to facilitate
breaking of bonds between reactants and formation of new bond to form products are
called effective collisions.The number of collisions per second per unit volume of the reaction mixture is
known as collision frequency (Z).COLLISION THEORYThus, in collision theory activation energy and
proper orientation of the molecules together determine the criteria for an effective collision and hence the
rate of a chemical reaction.Rate of reaction can be expressed as,Rate(r) =A + B → Products
(r) = ZAB e−Ea/RT
61) Describe about standard Hydrogen Electrode (SHE).
ans) The S.H.E. is defined as a piece of platinum metal, immersed in a unit-activity aqueous solution
of a protonic acid, and over whose surface hydrogen gas, at unit fugacity, is passed continuously.
These concentration choices make the electrode a standard electrode.
62) Limitation of crystal field theory ans) From the assumptions, that the ligands are point charges, it follows that anionic ligands should exert the
greatest splitting effect. But the anionic ligands actually are found at the low end of the spectrochemical
series.
63) Briefly explain the electrochemical processes involved in the rusting of iron
ans) Corrosion is a natural process that converts a refined metal into a more chemically stable form such as
oxide, hydroxide, carbonate or sulfide. It is the gradual destruction of materials by chemical and/or
electrochemical reaction with their environment. b) -According to the electrochemical theory of the rusting
of iron, oxidation reaction occurs at anode and reduction reaction occurs at the cathode. -At the anode, the
iron is oxidized to the ferrous ions and the electrons which are released move towards the cathode.
Oxidation: Fe(s)→Fe2+(aq)+2e−
reduction: O2(g)+2H2O(l)+4e−→4OH−(aq)
Fe2+(aq)+2OH−(aq)→Fe(OH)2(s)
64) Write any three applications of d - and
’f - block elements
ans) 1) They act as catalyst 2) They form alloys 3)Cu, AgAu are coinage metals 4) They have different oxidation
states (Any three)
65) What is Carbylamine test
ans) Carbylamine reaction: When a 1° amine is warmed with chloroform and alcoholic KOH to form isocyanide. This reaction is known as carbylamine reaction.
CH3−NH2+CHCl3+KOH→CH3−NC methyl isocyanide
This reaction is used as the test for 1° amine. 2° and 3° amine do not answer this test.
66) Discuss the structure of protein?
i) Primary structure
It gives an idea about the sequence of arrangement of amino acids in the protein chain.
ii) Secondary structure
It gives the manner in which polypeptide chain is folded. It gives the protein molecule the shape of presence of H-bonding between C=0 and N-H bond will increase the stability. According to this structure Polypeptide chain appears like a right-handed spring is called α-helix as shown below.
Explain graphically the variation of molar conductance of strong and weak electrolyte by taking Suitable example?
Variation of molar conductance of a strong and weak electrolyte.
For a strong electrolyte the molar conductance value increases with increase in dilution or increases with decrease in concentration of solution. Because when dilution increases more ions are produced and mobility of ion increases. The molar conductance of an electrolyte at zero concentration is known as molar conductance at infinite dilution or molar conductance at zero concentration and denoted by the symbol λm0 or λm∞.
67)MOLAR CONDUCTIVITY
"It is the conductance of a solution containing one mole of the dissolved electrolyte."
Chloroform is stored in dark coloured glass bottles. Why?
To prevent the decomposition of chloroform in presence of sunlight.
2CHCl3+O2→2COCl2+2HCl
(COCl2 is phosgene)
Give an example for a polyhalogeno compound. Write anyone use of it?
DDT. It is used as an insecticide.
DDT - The full form of DDT is Dichlorodiphenyltrichloroethane.
67) Cumene process – This is the industrial method of preparation of phenol. In this process, cumene is oxidized by air to cumene hydroperoxide. This intermediate on reaction with a dilute solution of an acid, produce phenol and acetone.
68) Preparation of alcohols from aldehydes and ketones using Grignard reagent (R-Mg-X)
ans)Grignard reagent react with aldehydes and ketones to form an intermediate which on hydrolysis produce alcohol.
69)Electrophilic substitution reactions of phenol
a) Nitration - With dilute nitric acid at low temperature (298 K), phenol yields a mixture of ortho and para nitrophenols. But With concentrated nitric acid, phenol is converted to 2,4,6-trinitrophenol(picric acid).
b) Bromination - When the reaction is carried out in CHCl3 or CS2 and at low temperature, monobromophenols are formed. But When phenol is treated with bromine water, 2,4,6-tribromophenol is formed as white precipitate.
70) Why is chloroform stored in dark, full bottles?
ans) To prevent its oxidation to poisonous phosgene (COCl2) under light:
2CHCl3 + O2 → 2COCl2 + 2HCl
71) Why are alcohols soluble in water?
ans) Alcohols form hydrogen bonds with water and can disturb water's
hydrogen bonding, thus they dissolve readily.
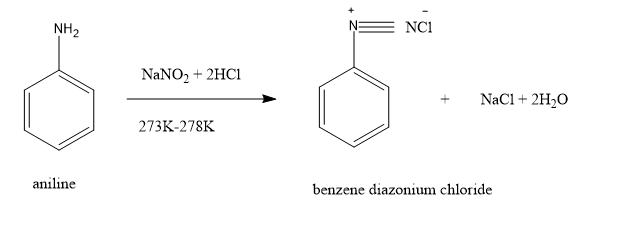
72) What is meant by diazotization?
ans) Diazotization is the reaction of an aromatic primary amine with nitrous acid
(generated from NaNO2 and HCl) at 273–278 K to form a diazonium salt.
73) Which vitamin is responsible for blood clotting?
ans) VIT K






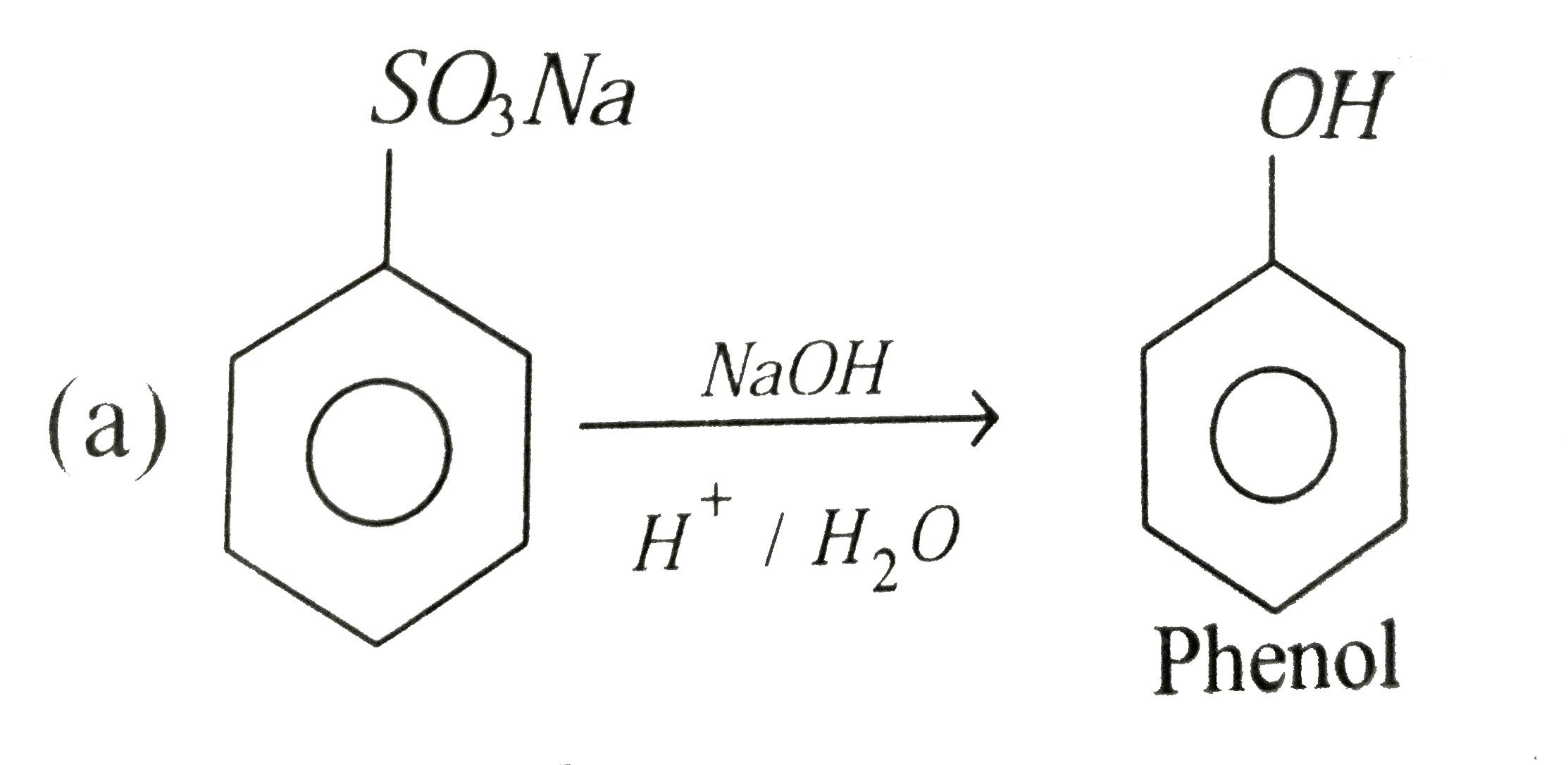






Comments
Post a Comment